By Tacconi Gianni and Michelotti Vania
Abstract
In the last 10 years, kiwifruit vine artificial pollination became a widespread practice useful to increase fruit quality. Kiwifruit size is directly proportional to the number of seeds, i.e., to the number of fertilized ovaries. However, artificial pollination efficiency depends on many parameters such as pollen quality (germinability, humidity, and conservation), pollination system (dry or liquid), coadjuvants, and flowering stage. Those parameters were well defined in Actinidia in recent studies, however, they remain quite undefined for other anemophilous pollinated trees such as olive tree, hazelnut, pistachio, and palm. In these plants, the flowers are very small and extremely numerous, so the pollination was difficult to study. In addition, there are incompatibility factors (genetic and physic), long lap time from pollination to fertilization, and alternate bearing, lower economic gain for these fruits, low agronomic input, and low innovation level in the field. All these aspects had reduced the application of pollination technique for these cultivations. The experiences developed in kiwifruit lead to define a new model crop fruit set that could be applied to anemophilous pollinated plants such as olive tree, where the fruit set are lower than 2%. The first experiences have shown a great potential and have encouraged the development of this technique.
1. Introduction
Pollination of crop plants is often the major requirement in achieving sufficient crop set [1, 2]. Insufficient pollination has been found to be one of the important causative factors of low yield and low quality in many fruit tree species [3]. Supplementary pollination is a valid support to increase productivity in crop species such as strawberry [4, 5], olive [6], kiwifruit [7, 8], almond [9, 10], pistachio [11, 12], hazelnut [13], macadamia [14, 15] and date palm [16, 17]. Artificial pollination leads also to increase final set, weight, kernel recovery, and, in many cases, fruit quality in terms of nutritional characteristics and shelf life [4]. Moreover, in olive tree, a greater pollination and fruiting cause a slower ripening of the drupes, and consequently harvest times are more suitable to the improvement of olive and oil quality. In many cases, natural pollination (both wind and bee) is often unsatisfactory or not constant in the years (Figure 1), because it can be affected by climatic factors, wrong synchronization of male and female flowering, and low attraction for bee since the absence of nectar in the flowers of wind-pollinated (anemophily) plants.

Kiwifruit artificial pollination was first studied by Dr. Hopping in 70 years [7, 18, 19] in New Zealand and in Italy, in collaboration with Dr. Cacioppo and Dr. Galimberti in Latina, in 1987 (Figure 2).

Kiwifruit (Actinidia chinensis var. deliciosa and A. chinensis var. chinensis) is a dioecious plant, and, in order to have good pollination, in orchard, there are female and male plants in 6:1 ratio. The pollination is mainly anemophilous (wind-pollinated), and the fruits size depends on the number of seeds: a 100 g fruit has more than 1000 seeds, and it is estimated that about 10fold pollen grains are necessary to reach this seed number [21, 22]. Also, an increase in male:female ratio to 1:1 (Figure 3) was not enough, in many cases, to optimize the pollination.

Moreover, in many specialized orchards, there are installed anti-hail net or plastic tunnel to protect the plants from climate injuries or from the bacterial disease Pseudomonas syringae pv. actinidiae [23]. These installations reduce the ventilation and indeed pollen movement. Furthermore, in yellow flesh kiwifruit (but also in green ones), often male plants were not planted in the orchard in order to have a higher yield (male occupy 16% of the surface) and an easier management of the plants (treatments for plant protection due the higher disease susceptibility of male, pruning, fertilization, and irrigation). In the cases where male plants are absent in the orchard, pollen are kept from specialized male orchards or buy on market (following plant protection rules to avoid diseases contaminations).
Kiwifruit artificial pollination is nowadays a consolidate technique to increase kiwifruit quality and size [8, 24]. However, pollination not always reacts with maximum efficiency: the results could change in different years and depend on the pollen harvesting system, pollen storage technique, pollination system (dry or wet), added substance to dilute pollen (dry or liquid) or to help the germination, pollination equipment, moment of pollination, floral stage of application, and economic impact of the operation (cost and gain). The analysis of these aspects could be applied to other crops and could be summarized in a flowchart where physiological aspects and human practices/decisions are integrated (Figure 4). Given the optimal pollen quality and optimal agronomical management (irrigation, fertilization, pruning), the results could vary in relation to the choice of the floral stage of intervention in relation to the type of pollination. In the reported studies, many parameters were analyzed alone and in interaction in different environments in Italy and for many years: pollen quality, pollination system, and flowering stage.
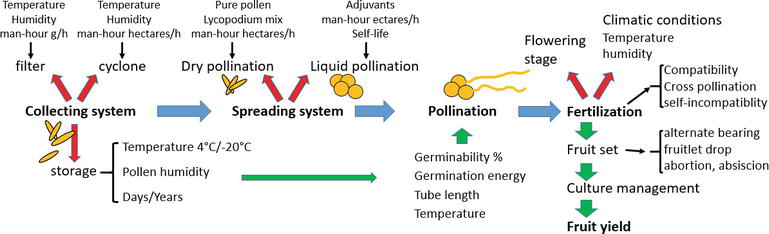
High-quality pollen is basic for good results: germinability, germination energy, and humidity were evaluated under different conditions of pollen harvesting, conservation at different temperatures and time of exposition at different temperatures, and manipulation before and during pollination in different pollination systems (dry and liquid).
The interaction of the pollination systems and the flowering stage were also evaluated.
Many aspects are in common with olive (Olea europaea L.) and can be applied to its pollination. Olive fruit set are very low, less than 2% of flowers, which in Northern Italy means about 10 kg of fruit per plant [25]. The main problems are self-incompatibility, scarce pollen from wild, wrong pollinator cultivars in the orchard, pollen quality and quantity, lack of coincidence of blooming period, and adverse climate conditions.
This observation leads to define kiwifruit pollination as a new model for crop fruit pollination that could be applied in other wind pollination (anemophily) trees such as olive tree, hazelnut, and pistachio that were studied but without a practical application (Figure 4) [6, 12, 13].
This chapter does not want be a review on biology of the pollination in kiwifruit and olive but an update of the research applied in the field supported by scientific data. Here, publicized works but also original researches data are reported.
2. Pollen quality for kiwifruit pollination
The first parameter that is considered to define the pollen quality is the germinability. Pollen could germinate but stop early in the tube growth: must it be considered right for pollination or not? Many grains germinate, but in different ways due to their different germination energies (germinability related to the time or germination tube length), and it is evident recording pollen germination under microscope (Figure 5 and related movie). Other parameters must be considered in order to evaluate pollen quality, as humidity and germination energy [26].
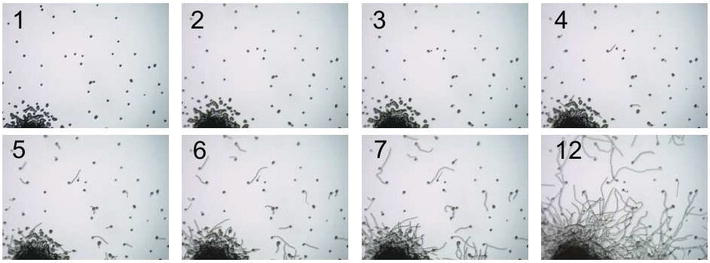
These parameters were evaluated under different conditions of pollen harvesting, conservation at different temperatures and different times, exposition at different temperatures, and manipulation before and during pollination. For example, stresses against pollen during harvest and manipulation result in decrease of germination energy more than decrease of germinability.
2.1. Materials and methods
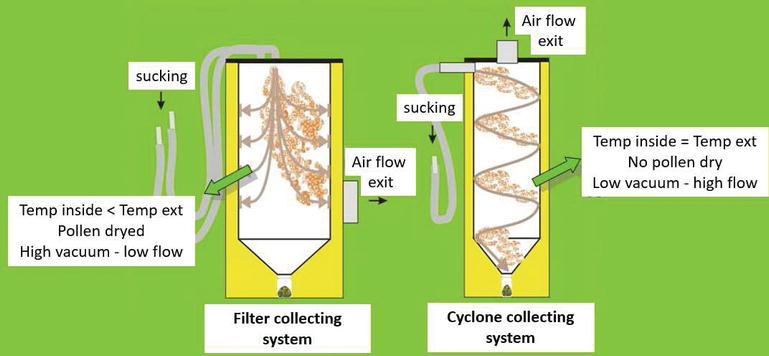
The experiment was performed on Actinidia deliciosa cv. Hayward (female) and cv. Tumuri (male) in the Tacconi Lorenzo’s farm in Verona (North Italy), on a plantation built in 1982, (T-bar system, 4.5 × 3 m) having a permanent corded male suspended in the middle of the inter-row (Figure 3). The pollen samples were collected in two seasons (2008 and 2009) having opposite conditions of high relative humidity (RH) and low temperatures and low RH and high temperatures, respectively. Pollen samples were collected with two different systems (Figure 6): filter separator (Aspir@Polline TR Biotac, Verona, Italy, www.biotac.it) and cyclone separator (AspiraPollineMini2 Biotac, Verona, Italy). The pollens were extracted from the machine and placed at 4°C every 45 min.
The germination temperature test was made with fresh Tumuri pollen (collected with Aspir@Polline TR Biotac) on standard substrate (sucrose 85 g/l, boric acid 0.5 g/l) by taking a photo of the same field of view under microscope every minute for 14 h. The temperatures considered were 18, 24, and 30°C. To test the in vitro germination conditions, the germination of two pollen samples (collected with Aspir@Polline TR and AspiraPollineMini2, Biotac) on different agar growth media was compared (Table 1). The germination was observed at intervals of about 1 h for 15 h under the microscope (Olympus BX51 microscope at 200 magnifications with Olympus DP50 camera). The germination was made at a constant temperature of 20°C in a growth chamber (Sanyo Gallenkamp PLC, Loughborough, UK) with RH 100% and with cold light. The germination was calculated as percentage of germinated pollen counting about 100 pollen grains in three different optical field; tube length was evaluated using UTHSCSA ImageTool software and reported as fold grain diameter (D, about 30 micron).
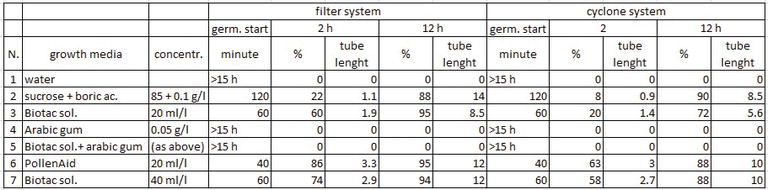
Table 1.
Percentage of germination and germination energy of pollen collected with different systems and germinated on various media.
2.2. Results and discussion
2.2.1. Germination: effect of temperature and growth media
Germination latency period and tube length are inversely proportional to the temperature of germination (Figure 7). During pollen application in field lower temperature is useful due the observation that the pollen tube length is higher if the germination appends at about 18–24°C, whereas at higher temperature (30°C), the germination stops early and tube length is lower. Moreover, the suspension of pollen in water must be sprayed before the germination starts, in practical within 40 min, to avoid pollen damage.
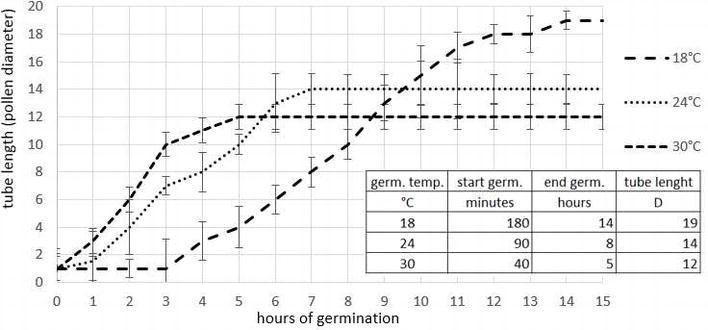
The different media used in vitro can give useful indications for pollen suspensions in the case of liquid pollination and for analysis. The different media showed a different percentage of germination and germination energy (given by start time of germination and final lengths of pollen tubes; Table 1).
The analyses carried out show how the result evaluation of germination could vary according to the growth media used and depending on the moment in which the observation is made. Furthermore, a media that is too nutritious (i.e., n. 6 and 7) could overestimate the real germination that would occur in vivo in field condition, whereas a less stimulating substrate (i.e., n. 2) would be more useful as it highlights any weakness (less germination energy). PollenAid and Biotac solution could be useful in liquid pollination because they encourage germination [26].
2.2.2. Pollen harvest systems, pollen humidity, and pollen viability
Different pollination machines are available in the market, and these fall in two categories basing on the separation system: filter and cyclone (centrifugation). The comparison of these systems in two different climatic conditions during pollen collection, in particular relative air humidity (RH), reveals some differences in the pollen quality. Regarding cyclone system, pollen RH increases with air RH increasing (RH), whereas in the case of filter system, pollen RH is about 10% independently to the air RH (Figure 8). This difference is due to the lower pressure inside the filter system and, therefore, lower temperature in comparison to external one, such that water vapor in the air is condensed and extracted.
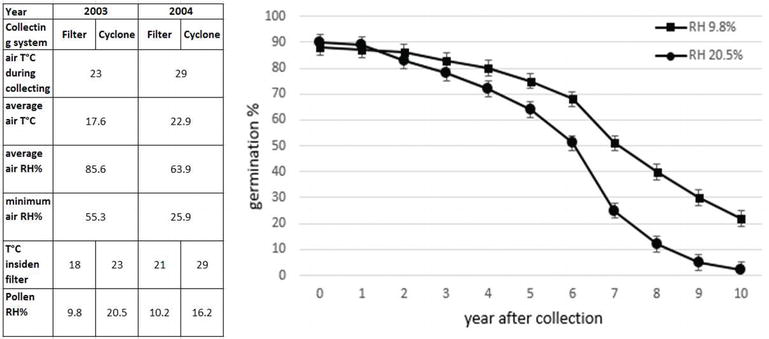
The humidity of the pollen is important for pollen long-term storage. One advantage of artificial pollination is the possibility to store pollen at −18°C for years maintaining its viability. For this purpose, pollen RH must be about 10–12%; in other ways its germinability decreases in direct proportionality with RH and years (Figure 8). For practical usage, it could be considered that pollen can be stored about 3 years if its humidity is low or after drying with silica gel.
2.3. Conclusion
The highest pollen quality was obtained when the pollen was picked up from the collecting machine frequently during the day (about every hour), to avoid any stresses, and stored at 4°C for no more than 7 days. Pollen can be stored at −18°C up to 3 years, better with low humidity or pre-dried to 10–12% with silica gel at 4°C. A recent method to estimate pollen viability was developed and is based on physical analysis of the single cells by impedance flow cytometry [27], and it could be interesting to compare the two methods especially during pollen storage.
3. The interaction between pollination systems and flowering stage in Actinidia
High-quality pollen is essential for good pollination, but pollination efficiency depends also on the equipments used and the time of application: dry pollination with pure pollen or diluted with lycopodium, liquid pollination in water suspension with adjuvants, handing application, or with mechanized tools [8]. In this paragraph, the interaction between pollination systems and the flowering stage will be elucidated, in order to understand which is the best flowering stage in relation to the pollination system adopted.
3.1. Materials and methods
All the experiments were performed on Actinidia deliciosa cv. Hayward in field condition with three repetitions per treatment, among 5 years (2009–2013) in three different environments: Cuneo (NO Italy), Verona (NE Italy), and Latina (Central Italy).
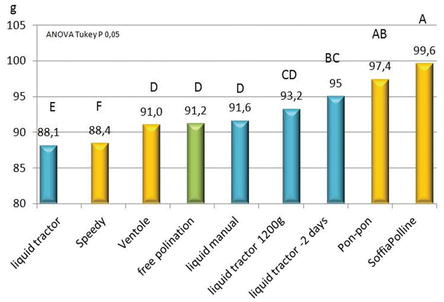
The comparing of pollination-systems (Figures 9 and 10) was conducted in collaboration with Agrion (Cuneo, www.agrion.it); the pollination was carried out with 90% of flowers at the stage of petal fall (with white pistils) with 600 g of pollen per hectare with a single-step distribution. The experimental design was a randomized block in standard orchards (female:male rate 1:6) with T-bar (Verona and Cuneo) and pergola (in Latina) trellis systems. The liquid distribution was 12 g/l of pollen in deionized water and 5 ml/l of activator PollenAid (Kiwi Pollen, New Zealand) for a total of 50 l/ha of water suspension. The machines used in the pollination system’s comparative test were reported in Figure 9.

The role of Lycopodium was evaluated in 2013 in Verona by comparing two systems of dry pollination with and without Lycopodium added. Lycopodium was added to pollen in dry pollination as inert in some machines like Speedy. Experimental design consisted of three theses (two rows each): pollination with the Soffi@Polline system with pure pollen, pollination with SoffiaPolline with pollen:Lycopodium mixture (55%:45%), and pollination with Speedy with pollen:Lycopodium mixture (55%:45%).
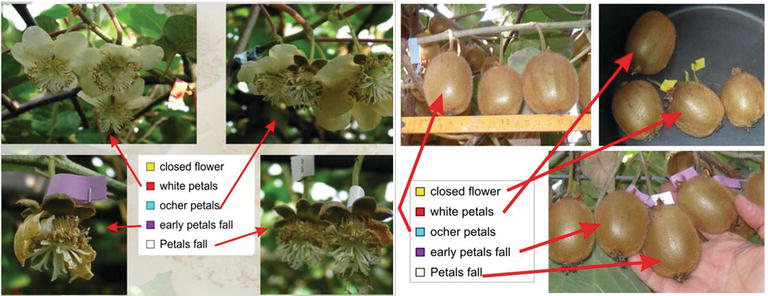
To understand the relation between flowering stage and the type of pollination, dry or liquid, just before pollination the flowers were labeled according to their flowering stage (Figure 11). The signed stages were, according to BBCH scale [28] are the following: closed flower (55–59), white petals (60–64), ocher petals (65–66), early petal fall (67), and petal fall (68) with most of the pistils white and stigmas viscous, just before pistils dry and ovary increasing (69). To understand the success of the pollination, about 100 fruits for three biological replicates were weighed at harvesting time (end of October). This experiment was repeated for 4 years (2010–2013) in Verona, Cuneo, and Latina using Soffi@PollineZ for dry pollination and “ElettroEASY” (or similar diaphragm pump) for liquid pollination.
3.2. Result and discussion
3.2.1. Comparison of equipments for pollination
Usually, the best pollination method considered is the manual method of pon-pon but because of its considerable employment of labor is rarely used in commercial orchards. As shown in Figure 10, it was overcome by Soffi@PollineZ pollinator, probably because with pon-pon some flowers were not touched, whereas the pollen powder blown reaches all the canopy. Analogously, with Speedy some flowers were not pollinated and, in addition the role of Lycopodium, will be analyzed in another experiment. Good results were also obtained with liquid pollination applied 2 days before the other when the 90% of flowers at the stage of petal fall. In other terms, it seems that liquid pollination could be better before petal fall. The following experiments will elucidate this aspect [8].
3.2.2. Role of Lycopodium in pollination
The low pollination rate observed using the pollen-Lycopodium mix Speedy machine (Figure 9A) is due to the drying effect of the Lycopodium on pistils and does not depend on the machine: the addition of Lycopodium to the Soffi@Polline gave the same results.
The fruit size obtained in the thesis pollinated with pollen-Lycopodium mixture was lower than the thesis pollinated with pure pollen: average weight 96 g with the addition of lycopodium, 106 g with pure pollen, and 75 g free pollinated fruit (data not shown). That result indicates that the presence of this inert may adversely affect fertilization, regardless of the distribution system.
3.2.3. Flowering stage
After the first evidence where liquid pollination appears more efficient before petal fall, the interaction between flowering stage and pollination system was investigated. Actinidia flowering is scalar, and the same flower is viable for about 4 days, in normal climatic condition, after that the pistil degenerates and starts the fruit set (Figure 11 and related movie). Regarding liquid pollination, the best results were at full bloom and at early petal fall (Figures 11 and 12A), whereas for dry pollination, the best results were reached at petal fall (Figures 1 and 12B) before pistil senescence [8]. The pollination efficiency is evident at the harvest but could be useful approximately within 30 days after pollination (see related movie). In this period there are endosperm cellularizations that define the final fruit size and are important to proceed with the thinning of the bad pollinated fruit to avoid loose of energy and favorite the growing of the best pollinated fruit.
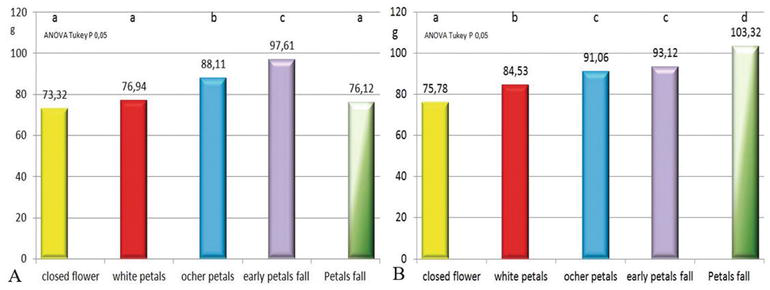
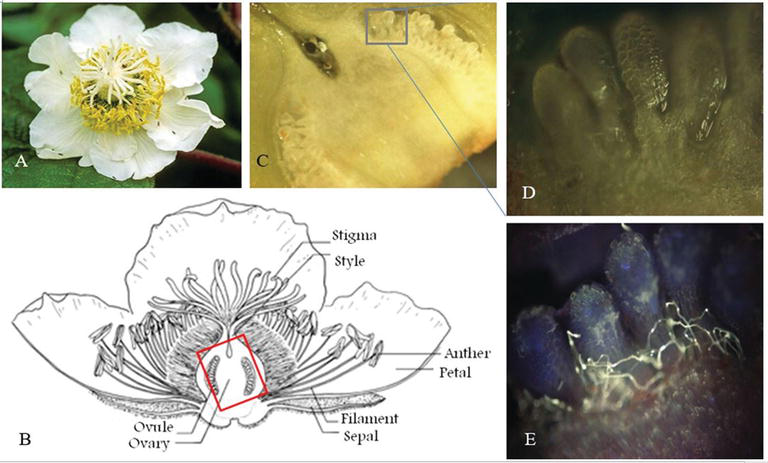
The flowering stage is easily described observing the petals, but it reflects more important aspect of the flower and in particular the pistil exudate, essential for pollen adhesion, germination, and the ovary receptivity. The pistil’s exudate production increases during flower life and, in cv. Hayward, is maximum at the petal fall stage (Figure 13). For yellow flash kiwifruit, it is less evident, and the flower has a lower self-life compared with Hayward and evolves within 1–2 days to late flowering stages. In this case, it is not possible to wait that all flowers reach the petal fall stage and the artificial pollination must be done every 1–2 days, depending on the climate conditions. It is notable also that, due to physical properties, the pistil’s exudate increases the pollen attached if it is powder, whereas decreases pollen adhesion if it is conveyed with water. Moreover, in dry pollination the fruit size is higher with respect to liquid pollination (Figure 12). This observation indicates indeed the receptivity of the ovules in the flower that is maximum just before pistil senescence (change from white to brown color) after petal fall. Often, early pollination leads to ovary-growing and pistil senescence even if not all ovules were fertilized, thus precluding the possibility of a complete pollination of the fruit. This phenomenon is visible observing the longitudinal section of the fruit (Figure 13) because, excluding phenomena of water stress, the ovule’s maturation is not simultaneous and starts from the petiole side to the tip side of the flower.

New histological analysis is under way in order to study the relationship between flower stage and ovary maturation. The process from pollen adhesion to fertilization could be observed in vivo by staining the pollen with aniline blue under UV light (Figures 14 and 15).
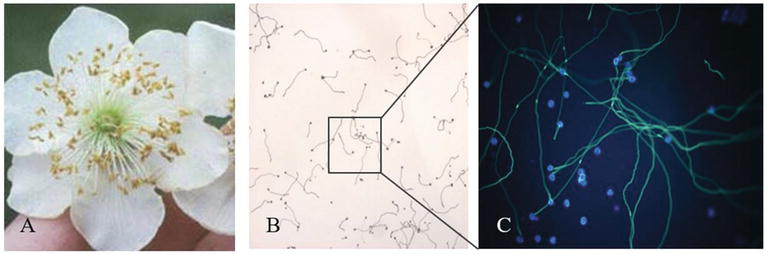
In Actinidia, fertilization appends within only 6 h after pollination (Figure 15), and this aspect facilitates the study of the relation between the moment of pollination and the flower stage. The Actinidia floral biology could be useful as model of wind-pollinated trees in field condition.
3.3. Conclusion
Kiwifruit artificial pollination, in conventional orchard, increases the production up to 30% (Figures 10 and 12) due to bigger fruit size. Pollen is collected from male plants, and to maintain its viability is necessary to avoid high temperature and high humidity. In practice it is picked up from the collecting machine every 45 min and stored at 4°C for ready usage (up to 7 days) or for long storage at −18°C (3 years if its humidity is lower than 12%) (Figure 8). Both liquid and dry pollinations are effective if done at the right flowering stage: liquid pollination not later than early petal fall stage, dry pollination, with pure pollen, at petal fall stage (in cv. Hayward) when the pistils exudate is maximum (Figures 11–13), at early morning with high air humidity. In order to pollinate early and late flowering flowers, the pollination must be done in two steps or more in particular in yellow flash kiwifruit. In any cases, dry pollination seems to be most suitable because it is applied when the number of mature ovaries in the flower is maximum.
